Oral Mucositis
Oral mucositis, a common and often debilitating inflammation and ulceration that occurs in the mouth as a side-effect of certain cancer treatments, afflicts approximately 450,000 patients each year in the United States and affects the course and outcome of cancer therapy. In addition to increasing patient risk and discomfort, oral mucositis is also associated with increased treatment costs of up to $25,000 per patient. Despite these numbers, there are no effective pharmacological therapeutic options for this condition and treatment today is mostly focused on pain management.
Brilacidin has shown antibacterial, anti-biofilm and anti-inflammatory properties in various pre-clinical studies. We believe that the combination of these attributes contribute to the efficacy that brilacidin has shown in animal models of oral mucositis. In addition, we believe there is a significant unmet medical need and pharmacoeconomic rationale for evaluating brilacidin in the future as a therapeutic topical drug to treat cancer patients with oral mucositis.
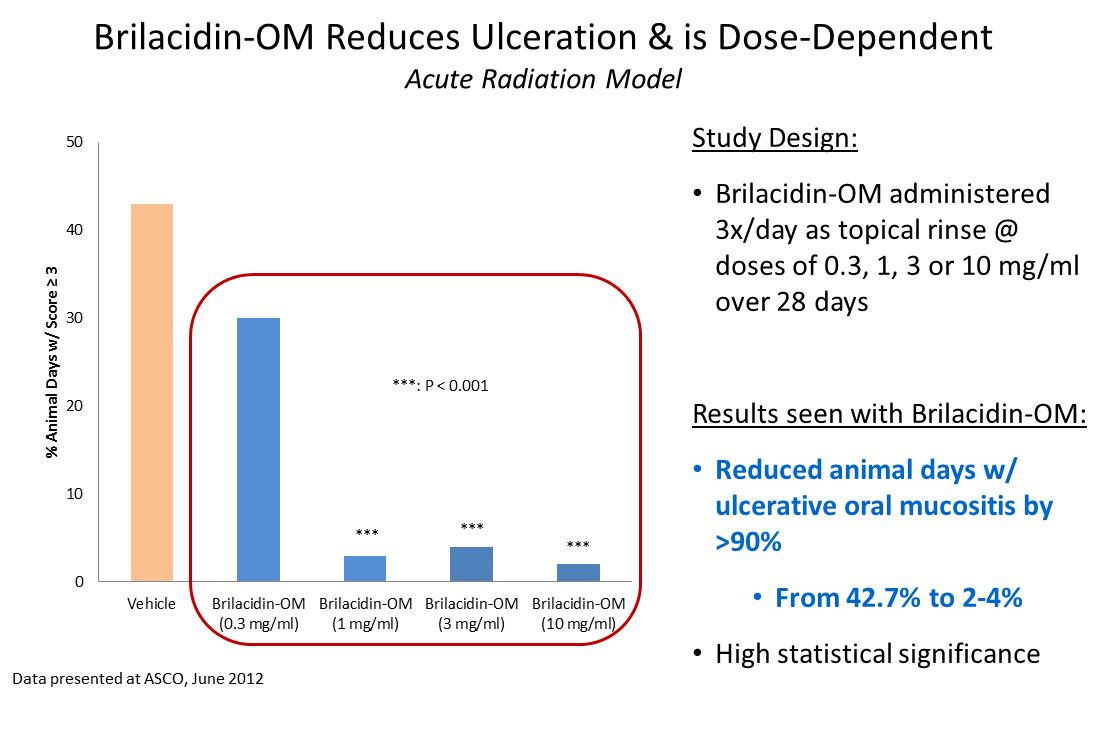
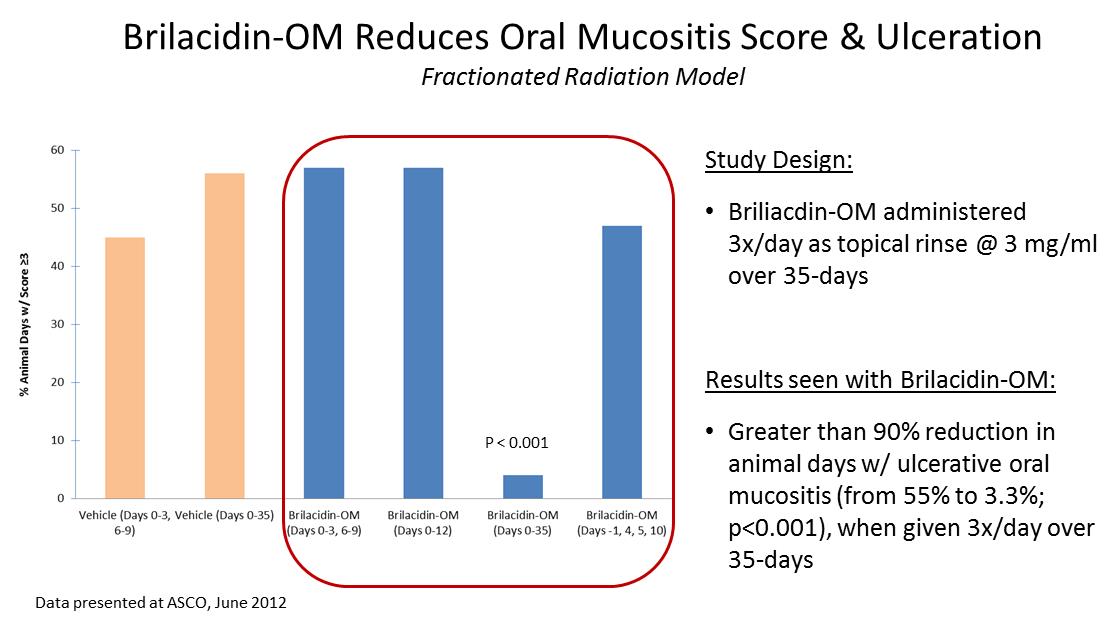
Brilacidin was tested in two animal models where oral mucositis was induced by a single high dose or multiple lower doses of radiation to mimic radiation therapy used commonly in certain types of cancer. The results from both models showed that brilacidin when administered as an oral rinse significantly reduced the severity and duration of ulcerative mucositis. These studies were conducted at Biomodels by Stephen T. Sonis, DMD, DMSc, Chief Medical Officer at Biomodels, Professor of Oral Medicine at Harvard’s School of Dental Medicine. The time course of efficacy seen in these animal models indicates that immunomodulatory and/or anti-inflammatory activities of brilacidin may be important underlying mechanisms of action for its efficacy in oral mucositis.
Clinical Development
Cellceutix Investigational New Drug (IND) Application Becomes Effective, Selects First Site for Phase 2 Clinical Trial of New Treatment for Oral Mucositis
https://clinicaltrials.gov/ct2/show/NCT02324335?term=cellceutix&rank=2
FDA Grants Fast Track Designation to Cellceutix’s Brilacidin-OM for Oral Mucositis
BEVERLY, MA-(Marketwired - November 25, 2015) - Cellceutix Corporation (OTC: CTIX) (the “Company”), a clinical stage biopharmaceutical company developing innovative therapies with oncology, dermatology, anti-inflammatory and antibiotic applications, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for Brilacidin-OM, an oral rinse formulation of the Company’s novel defensin-mimetic Brilacidin, for the prevention of oral mucositis. Oral mucositis, a common and often debilitating inflammation and ulceration that occurs in the mouth as a side-effect of certain cancer treatments, afflicts approximately 450,000 patients each year in the United States and can affect the course and outcome of cancer therapy. There are no FDA approved drugs for the prevention of oral mucositis.
In an ongoing Phase 2 trial, Brilacidin-OM is being evaluated for its safety and efficacy in preventing oral mucositis in patients undergoing chemoradiation for the treatment of head and neck cancer. Seven clinical sites and two satellite sites are currently enrolling patients in the trial, with additional sites expected to open enrollment in the coming weeks.
The FDA established the Fast Track Designation process to facilitate the development, and expedite the review of, drugs that have the potential to treat serious and life threatening conditions and fill an unmet medical need. Drugs developed under the Fast Track program are afforded increased access to the FDA and could qualify for other programs to expedite development, including priority review and accelerated approval.
Animal Models of Oral Mucositis
In the acute radiation model, brilacidin was applied as a topical rinse three times daily, at 0.3, 1, 3 or 10 mg/ml over a 28 day treatment regimen (n=10), following a single high dose of radiation to the buccal cheek pouch (40 Gy). Brilacidin exhibited efficacy in a dose-dependent manner. At the three highest doses, brilacidin significantly reduced peak mucositis scores and daily mucositis scores on 9 to 10 of the 10 evaluation days when mucositis was evident ( p<0.001). Brilacidin also reduced the number of animal days with ulceration from 42% in vehicle treated animals, to <5% in the three highest dose groups (p<0.001), a reduction of approximately 90%.
In the more stringent fractionated radiation model, lower doses of radiation (6.5 Gy/dose) were administered on days 0, 1, 2, 3, 6, 7, 8 and 9, targeting the left buccal pouch mucosa. The total cumulative radiation dose is higher in the fractionated model (52 Gy) than in the acute model (40 Gy). Brilacidin was given topically three times a day at 3 mg/ml over a 35- day regimen beginning on the first day of radiation exposure. Brilacidin significantly reduced the daily mucositis scores prior to peak mucositis and throughout the remaining course of treatment (p<0.001), and reduced the number of animal days with ulcerations from 55% in vehicle treated hamsters to 3.3% (P<0.001), a reduction of approximately 94%.